You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Blue Star OG pheno hunt, PPK style
- Thread starter greyfader
- Start date
i want to discuss the seeds a little. Josh sent me 50 to hunt through. 48 germinated within 2 days for a 96% germ rate.
i took the first 36 to develop 1/2" tails and started them in wick-fed solo cups with perlite.
about 2.5 weeks there and the best 14 went into 2.5-quart wick-fed perlite containers.
approx another 2.5 weeks and then the best 12 into these 4 gal totes.
i took the first 36 to develop 1/2" tails and started them in wick-fed solo cups with perlite.
about 2.5 weeks there and the best 14 went into 2.5-quart wick-fed perlite containers.
approx another 2.5 weeks and then the best 12 into these 4 gal totes.
Attachments
we are about a week from transplant from 2.5 quart to 4 gal containers. they were passively fed from the bottom with a little hand watering the tops in the 2.5 qt containers then into the active ppk system. they are responding nicely.
Attachments








just a little pictorial showing how the system is made. the float valve controls the water level.
the surface of the medium is 13" above the floor.
the support containers below each plant are 7" high. the solution level is 3".
This leaves a 4" "air gap between the bottom of the plant container and the solution.
the tube protruding into the solution is 6" long and the bottom 2" of it stays wet allowing capillary rise to feed the plant from below.
this represents a 24/7 hydraulic hook-up between the plant and nutrient solution.
the top watering apparatus is timed by a repeat cycle timer to fire the 700 gph pumps (one in each pool) on a schedule. right now they are getting about a quart in 20 seconds every 2 hours.
there exists in every plant container, immediately after a watering event, a perched water table.
this is a body of water held up by the medium against the force of gravity. this PWT can only be eliminated by transpiration and evaporation.
this is because of waters cohesion and adhesion. cohesion is waters attraction to itself and adhesion is waters attraction to other substances.
this is why you cannot water a conventional container 12 times a day. the plant will drown if you do not allow it to use up the solution in the bottom of the container.
the PPK device positively drains the PWT from the medium immediately after each pulsed delivery of solution.
this is because the perched water table is moved from the plant container down into the tailpiece tube.
the perched water table exists at the same height in any shape container. it is a function of particle size. most plant growing media will support a perched water table. the finer the particle size the greater the volume of the PWT.
so by moving the PWT from the main plant container down into the capillary rise, drainage tube we radically reduce the volume of the PWT. it will still exist but it is greatly reduced in volume.
this, in turn, keeps most of the roots out of the saturated zone.
the medium here is chunky perlite which is loaded first. this material and sizing makes an extremely aerated media structure that has an air-filled capacity of about 30-35%.
i then add 1 lb worm castings and 1/2 cup of diatomaceous earth and work them into the top 2". this adds a substantial CEC or cation exchange capacity and retains more moisture in the top of the medium.
oxygen is the turbocharger in this system.
the roots sit in an ideal medium for growth.
the root zone is an interface of roots, water, air, and nutrients. this device keeps the ideal balance between these elements of the rhizosphere.
this system is a closed-loop, recirculating system.
i do not remove any solution from the system during a grow. it is all input only.
in this system the nutrient solution could be completely anaerobic and the plant will still grow just fine.
this is because the plant is deriving it’s oxygen from the ambient air instead of water.
two theoretical identical containers, one full of water and one full of air, both at sea level at 68f.
the container with air will have 23,300 times more free oxygen molecules than the water filled one at maximum saturation.
the act of watering stimulates plant growth by removing old, depleted gas by displacement and drawing in new fresh gas as the solution drains from the container.
we have found that this combination of a 24/7 sub-irrigation with a timed, quantified, pulsed delivery of solution from the top keeps the medium in an ideal band of parameters for a longer period of time than traditional containers.
this device, used in this fashion, has a synergistic effect on plant growth.
it is also extremely redundant with almost no failure points. if the electricity goes off the plant will simply back-feed from the reservoir and will grow just fine.
it will not produce as large of a plant if operated passively only but it will be a beautiful, healthy plant anyway.
this device allows the operator to fine-tune the moisture content in the medium using three tools.
first, the extent of the capillary rise can be controlled by the float valve. By raising or lowering the solution under the plant you can control the capillary rise and therefore the moisture distribution curve which is set by gravity, drier at the top and wetter at the bottom.
second, using the repeat cycle timer, you can adjust the duration and therefore volume of the pulsed top watering event.
third, again using the timer, you can adjust the interval between watering events.
by observing the plant and adjusting these 3 tools you can keep the root zone in an ideal state.
this device eliminates ph swings caused by nutrient buildup because the root zone is never allowed to dry down and the microscopic movement of the solution ensures that diffusion is always working.
well, that’s enough for now.
Last edited:
thank you! this is just another version of the PPK flow pattern. it's something i came up with in the summer of 2009 and i have been experimenting with it since. it is not a specific build but rather a flow pattern.Very interesting set up and explanation. Very keen to see this run throughout. Love seeing new ways
i have plumbed 10k sq ft rooms with it and i have friends using the idea in tents. it is the only closed-loop recirculating system that i know of that works outdoors in 100f heat.
i have set up 6 24x100 greenhouses for cbd production in tennessee and there are greenhouse setups in Oregon, California, and Washington state using it.
one in palm desert, which is a hot place.
i have multiple long threads on the idea at icmag under the handle Delta9nxs.
today marks 2 weeks since transplanting the first group into these 4 gal containers.
here are a few shots to show progress.
here are a few shots to show progress.
Attachments
today is 3 weeks since the transplant from 2.5 quart containers.
just a few pics to show progress.
i have rearranged them according to height to better manage the application of light.
i have taken one set of clones and will take one more before i flip them.
i have started thinning the largest fully expanded fan leaves.
they are beautiful, trouble-free plants. very vigorous.
getting some interesting stem rubs. i'm taking notes on my impressions.
the pic of the meters shows the current reservoir conditions.
i have not removed any solution from the system, it is all input.
just a few pics to show progress.
i have rearranged them according to height to better manage the application of light.
i have taken one set of clones and will take one more before i flip them.
i have started thinning the largest fully expanded fan leaves.
they are beautiful, trouble-free plants. very vigorous.
getting some interesting stem rubs. i'm taking notes on my impressions.
the pic of the meters shows the current reservoir conditions.
i have not removed any solution from the system, it is all input.
Attachments
so, i guess a little explanation of my absence is called for. i just went through a series of medical issues that pretty much deprived me of sleep for about 4 weeks. i turned into a zombie and was physically unable to do all the little things that had to be done on time to flower at 4 weeks of veg. they were flowered at 40 days and, of course, had overgrown the space.
today is 21 days of flower and about the end of the stretch. i have multi-topped and thinned heavily. i still have to do some larf removal.
i just now switched out the 5000k bulbs for the 2700k plus the incandescents. i had planned to do it at 2 weeks of flower instead of 3.
so all of the growth in these pics occurred with 5000k bulbs.
there are 66 2700k and 6 25-watt incandescents for a total of 1074 watts in each fixture. the incandescents are roughly centered over each plant.
it's been 8 weeks and 5 days since i started this grow and no solution has been removed from the reservoirs. all input only.
i have both lights hard-tied to the top of the frame and do not have any more headspace but i should be good.
under the square fixture, i'm getting pretty uniform par readings between 1150-1250 umols.
and, under the octagonal one, it's between about 1200-1300 umols.
it's a good thing i'm using the 8-16 photoperiod as 8 hours of 1200 umols is 34.56 moles per period.
i've got a little tip burn as i let the feed reservoir run dry a couple of times and the ec crept up to about 2.6 for a day or two.
i will try to update on some kind of schedule from now on.
i'm getting some fairly powerful stem rubs now and some slight differences in smell.
i'v got full sets of clones of 8 of the 12.
today is 21 days of flower and about the end of the stretch. i have multi-topped and thinned heavily. i still have to do some larf removal.
i just now switched out the 5000k bulbs for the 2700k plus the incandescents. i had planned to do it at 2 weeks of flower instead of 3.
so all of the growth in these pics occurred with 5000k bulbs.
there are 66 2700k and 6 25-watt incandescents for a total of 1074 watts in each fixture. the incandescents are roughly centered over each plant.
it's been 8 weeks and 5 days since i started this grow and no solution has been removed from the reservoirs. all input only.
i have both lights hard-tied to the top of the frame and do not have any more headspace but i should be good.
under the square fixture, i'm getting pretty uniform par readings between 1150-1250 umols.
and, under the octagonal one, it's between about 1200-1300 umols.
it's a good thing i'm using the 8-16 photoperiod as 8 hours of 1200 umols is 34.56 moles per period.
i've got a little tip burn as i let the feed reservoir run dry a couple of times and the ec crept up to about 2.6 for a day or two.
i will try to update on some kind of schedule from now on.
i'm getting some fairly powerful stem rubs now and some slight differences in smell.
i'v got full sets of clones of 8 of the 12.
Attachments
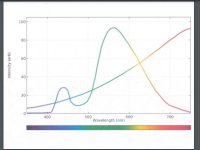
thank you! the incandescents are part of an experiment to add dark red, far red, and a little infrared to the spectrum in a cost-effective way. below is a composite graph i put together showing a typical warm white overlaid with a tungsten incandescent.Sorry to hear about your recent health issues greyfader; glad you're feeling better and great to have you back
Wondering what is the purpose of the 6 incandescents ?
Is the 8/16 schedule to try to have them finish faster and limit their growth at this point ?
the incandescent has more dark red than the sun as a portion of par.
i'm trying to induce more flower extension than i can get with a 2700k warm white led. increase flower weight and size.
the 8/16 schedule is to deliver the total moles per diurnal period in a shorter time frame than 12/12. i've done 4-5 grows with this schedule so far and think i'm getting a danker plant with better terpene retention.
some very experienced led growers are getting excellent results with about 750 umols for 12 hours which is 32.4 moles per period.
i'm using 1200 umols for 8 hours which is 34.56 moles per period.
Attachments
notdezmond
In Bloom
Glad to hear you’re doing better. Question for ya, i think dr bruce bugbee recommends 60 moles (1500umol) is optimum. Are you running that because of co2 limitations?
Excuse my ignorance but I’m just recently starting to feel the need to focus on the lighting optimization aspect. I have a hard enough time not over watering lol
Everything is looking great in here btw. Love it. And I appreciate your in depth entries.. I wish I could present all my thoughts in such a way lol
Excuse my ignorance but I’m just recently starting to feel the need to focus on the lighting optimization aspect. I have a hard enough time not over watering lol
Everything is looking great in here btw. Love it. And I appreciate your in depth entries.. I wish I could present all my thoughts in such a way lol
Glad to hear you’re doing better. Question for ya, i think dr bruce bugbee recommends 60 moles (1500umol) is optimum. Are you running that because of co2 limitations?
Excuse my ignorance but I’m just recently starting to feel the need to focus on the lighting optimization aspect. I have a hard enough time not over watering lol
Everything is looking great in here btw. Love it. And I appreciate your in depth entries.. I wish I could present all my thoughts in such a way lol
first, thank you for all the kind words.
doctors Bugbee and el sohley at the University of Mississippi found that the highest rate of photosynthesis in cannabis occurs at 1500 umols. the rate increases linearly with increasing light until you hit 1500 umols and then plateaus until 2000 umols. after 2000 umols the rate of photosynthesis drops straight down like it's falling off a cliff.
these studies were done by using integrating spheres with leaf fragments, not whole plants for 12 hours.
below is the famous jim faust dli map. these are average mole counts per day.
i grew with hps bare bulbs vertically oriented in a checkerboard pattern for 21 years. i would run these bare bulbs as close as 16" to get 1500 umols at the closest part of the plants. 1500 umols for 12 hours is 64.8 moles.
at about 8 hours into the period, i and other growers using this level of light noticed what became known as the "wall".
at this point the plants would begin showing light avoidance by drooping fan leaves or twisting the leaf ends sideways to the light reducing the total light the plant receives. the sideways twisting reminded me of trimming a sail that is getting too much wind.
i think this is because the plant has produced more synthase than it can process in a timely fashion. photoinhibition occurs until the plant processes the synthase.
as we see from the map, few places get 64.8 moles a day. tomatoes are also high-light plants. there are greenhouses in northern latitudes getting around 30 moles per day either naturally or with supplemental lighting that are producing heavy yields.
in high light areas like California or Arizona most outdoor growers that i have met use a partial shade cover cloth to reduce the dli by 35-50%.
i think we should be focused on moles per day instead of an instantaneous umol reading.
i know i'm growing a better quality plant with leds in general. the potency is there and the terpenes are much better than with hps.
maybe overdriving the plant with a sustained high-intensity flow of light degrades terpenes similarly to high heat.
i have done some very high dli grows in the last year or two just to see what would happen. 1500-2000 umols for 12 hours.
what i saw was shocking. i was expecting the largest flowers to be at the top but they appeared stunted compared to the flowers 6-8" further down the branch. this is with leds and not hps but that's another subject.
so i think i caused this with too much light at the tops.
about co2. i see a lot of folks thinking that as the light flow increases so should the co2 concentration in the space. i don't believe this is true.
co2 is consumed by the plant on a per-unit basis. you can't force more co2 into a plant than it's using at any given metabolic rate.
i prefer sealed rooms for a bunch of reasons and have tried supplemental co2 all the way up to 1500 ppm. i did not get better results than i did with 750-900 ppm. in a large sealed room with a bunch of big plants you want to supply more than ambient levels just in case they suck it all out of the air leaving below ambient levels. it's like insurance because plants in this scenario can really take it up.
but, in a room that is open to ambient co2 you just need enough airflow to make sure you do not go below ambient regardless of demand.
i have grown huge, heavy plants in both situations. and we have seen outdoor growers produce whopper plants with huge nugs using ambient co2 levels.
i will show a few plants i have grown using sealed and open rooms.
the first 2 are from my last effort with hps in oregon in a sealed room using 750-900 ppm co2.
the next 2 are from the 10k sq ft cbd facility i designed and built in Nashville. also tightly sealed using 750-900 ppm co2 and LEDs.
the last 3 are a plant grown in an open room using hps and just a lot of airflow.

doctors Bugbee and el sohley at the University of Mississippi found that the highest rate of photosynthesis in cannabis occurs at 1500 umols. the rate increases linearly with increasing light until you hit 1500 umols and then plateaus until 2000 umols. after 2000 umols the rate of photosynthesis drops straight down like it's falling off a cliff.
these studies were done by using integrating spheres with leaf fragments, not whole plants for 12 hours.
below is the famous jim faust dli map. these are average mole counts per day.
i grew with hps bare bulbs vertically oriented in a checkerboard pattern for 21 years. i would run these bare bulbs as close as 16" to get 1500 umols at the closest part of the plants. 1500 umols for 12 hours is 64.8 moles.
at about 8 hours into the period, i and other growers using this level of light noticed what became known as the "wall".
at this point the plants would begin showing light avoidance by drooping fan leaves or twisting the leaf ends sideways to the light reducing the total light the plant receives. the sideways twisting reminded me of trimming a sail that is getting too much wind.
i think this is because the plant has produced more synthase than it can process in a timely fashion. photoinhibition occurs until the plant processes the synthase.
as we see from the map, few places get 64.8 moles a day. tomatoes are also high-light plants. there are greenhouses in northern latitudes getting around 30 moles per day either naturally or with supplemental lighting that are producing heavy yields.
in high light areas like California or Arizona most outdoor growers that i have met use a partial shade cover cloth to reduce the dli by 35-50%.
i think we should be focused on moles per day instead of an instantaneous umol reading.
i know i'm growing a better quality plant with leds in general. the potency is there and the terpenes are much better than with hps.
maybe overdriving the plant with a sustained high-intensity flow of light degrades terpenes similarly to high heat.
i have done some very high dli grows in the last year or two just to see what would happen. 1500-2000 umols for 12 hours.
what i saw was shocking. i was expecting the largest flowers to be at the top but they appeared stunted compared to the flowers 6-8" further down the branch. this is with leds and not hps but that's another subject.
so i think i caused this with too much light at the tops.
about co2. i see a lot of folks thinking that as the light flow increases so should the co2 concentration in the space. i don't believe this is true.
co2 is consumed by the plant on a per-unit basis. you can't force more co2 into a plant than it's using at any given metabolic rate.
i prefer sealed rooms for a bunch of reasons and have tried supplemental co2 all the way up to 1500 ppm. i did not get better results than i did with 750-900 ppm. in a large sealed room with a bunch of big plants you want to supply more than ambient levels just in case they suck it all out of the air leaving below ambient levels. it's like insurance because plants in this scenario can really take it up.
but, in a room that is open to ambient co2 you just need enough airflow to make sure you do not go below ambient regardless of demand.
i have grown huge, heavy plants in both situations. and we have seen outdoor growers produce whopper plants with huge nugs using ambient co2 levels.
i will show a few plants i have grown using sealed and open rooms.
the first 2 are from my last effort with hps in oregon in a sealed room using 750-900 ppm co2.
the next 2 are from the 10k sq ft cbd facility i designed and built in Nashville. also tightly sealed using 750-900 ppm co2 and LEDs.
the last 3 are a plant grown in an open room using hps and just a lot of airflow.

Attachments
notdezmond
In Bloom
I appreciate the response! Damn that’s some sick pictures of Your past projects. Also you’ve given me much more to investigate haha thank you brother
it’s time for the weekend update. not much going on. the ppk device has been operating itself.
as far as the pheno hunt is concerned i look for not only potency and terpene profile but plants that are morphologically better suited for production. tight to medium node spacing, interiors that are not real “busy” so that i don’t have to spend a lot of time and effort cleaning them out.
in this particular grow, because of the overgrowth and subsequent butchery, i have given up on the morphologic part of the hunt and am just trying to finish choosing for potency and terps.
this is the end of week four of flower and i took 6 copies of each of the 8 i decided to clone. they are now stretched out too but have many nice shoots to choose from so sometime this coming week i will take another set that should be about ready to go for the next phase of the hunt.
i plan to run all 8 of the cloned plants next time not the 12 you see here. unless i find one or two that just don’t have the terps. but, i think this is highly unlikely since they all reek right now.
so the next run with 8 will include the morphological search as well as a more refined potency and terp search. hopefully i will be able to do everything on time. i’m feeling much better now.
just a few canopy shots to show progress. still no solution has been removed from the pool reservoirs under the plants. input only and today it read 1180 ppm at the .5 conversion or close to ec 2.4 and the ph is holding steady at about 6.0. 68 days of input only.
some are showing larger terminal flowers but it doesn’t mean anything because about 2/3 of the shoots have been topped. next time i’ find out which ones throw the most weight.
as far as the pheno hunt is concerned i look for not only potency and terpene profile but plants that are morphologically better suited for production. tight to medium node spacing, interiors that are not real “busy” so that i don’t have to spend a lot of time and effort cleaning them out.
in this particular grow, because of the overgrowth and subsequent butchery, i have given up on the morphologic part of the hunt and am just trying to finish choosing for potency and terps.
this is the end of week four of flower and i took 6 copies of each of the 8 i decided to clone. they are now stretched out too but have many nice shoots to choose from so sometime this coming week i will take another set that should be about ready to go for the next phase of the hunt.
i plan to run all 8 of the cloned plants next time not the 12 you see here. unless i find one or two that just don’t have the terps. but, i think this is highly unlikely since they all reek right now.
so the next run with 8 will include the morphological search as well as a more refined potency and terp search. hopefully i will be able to do everything on time. i’m feeling much better now.
just a few canopy shots to show progress. still no solution has been removed from the pool reservoirs under the plants. input only and today it read 1180 ppm at the .5 conversion or close to ec 2.4 and the ph is holding steady at about 6.0. 68 days of input only.
some are showing larger terminal flowers but it doesn’t mean anything because about 2/3 of the shoots have been topped. next time i’ find out which ones throw the most weight.
Attachments
Similar threads
- Replies
- 29
- Views
- 2K