Hey folks. My apologies for being inactive. I've been hyper focused on figuring out my garden and I think after 85 days of struggling, that I may have a solution in place.
On my last update from Dec 26th I switched nutrient lines to Canna A&B and that lasted about one week. I realized my heart wasn't set on using Canna. I was just too familiar with dry powders that I didn't want to go back to liquid nutrients. I was casually browsing
www.customhydronutrients.com to get some information on fulvic acid when I stumbled across a 5-11-26 nutrient they re-package and sell. Very similar to Jacks 5-12-26, but slightly less P, and about 1/2 the sulfur content. The price tag was so cheap that I had to order a container.
Upon arrival I noticed the powder is
very fine in comparison to the Jacks 5-12-26 I had been using. So I mixed up a batch using a magnetic stir bar and discovered this stuff solubilized quickly, clean, and did not leave sediment at the bottom. Half strength of
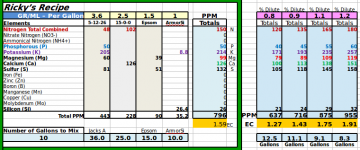
1.8 | 1.25 | .60 came out to EC 1.1 and PH landed at 5.5-5.6 consistently, so just a few drops of PH up was needed.
Around this time I also started watching some lessons from Bruce Bugbee on YouTube, a scientist and professor at Utah State University who oversees their cannabis program. One of the takeaways was he doesn't exceed an EC of 1.3. Albeit he grows in peat moss + vermiculite, a lot of the same principals apply in coco.
So I decided I would keep the EC of my vegging plants at 1.3 and young plants at 1.0. I would be cognizant of how much and how often I watered, and really observe how the plants reacted. About 2 weeks in and the plants began showing noticeable improvements in their color and posture. I was using the 5-11-26 in conjunction with 15-0-0 calnit and epsom salt, just like Jacks 321.
I then decided (out of left field) to order a 2lb bag of Jacks 5-12-26 from Amazon. I wanted to see for myself the differences between the 5-11-26 from customhydro, the old Jacks 5-12-26, and the newer bag of 5-12-26.
Upon arrival I was surprised. The powder in the new bag of Jacks 5-12-26 is completely different from the old. No big chunks, no deep red tint, no adulterations. Just a clean powder. I pulled out the magnetic stirrer and mixed up a batch, and to my surprise it solubilized completely. No chunks in the bottom. Same similar red tint to the water but everything dissolved.
I decided to run a few tests and mix a few batches up to see how the EC reads. This formula is a little weaker, and after looking at the bag it says to mix what would equal 4 grams per gallon instead of the old 3.6 grams per gallon.
Needless to say I decided to put the 5-11-26 from customhydro on hold and work with this new bag of Jacks 5-12-26. So far the results have been good. The plants are responding well. They still have some leaf discoloration from before, but everything is looking to be heading in the right direction. I did decide the to bump up the EC from 1.3 to 1.5 yesterday, as I felt they are looking a little light, but I wont be going any higher than 1.5 unless they really show me they need it.
Old (bad) Jacks 5-12-26
 New bag Jacks 5-12-26
New bag Jacks 5-12-26