The BX2 were freebies in the last drop at GLG.how can I get some of those BX GC
Guest viewing is limited
- You have a limited number of page views remaining
- 6 guest views remaining
- Register now to remove this limitation
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Schwaggy P's Random Stuff
- Thread starter Schwaggy P
- Start date
?what in all that is good in this world is Hippy Slayer
(RKS x Dirty Hippy) --> [ RKS x (Afgooey x Blockhead) ]I believe that Hippy Slayer is a Bodhi strain. Dirty Hippy x Road Kill Skunk
It's been unavailable from Bodhi for awhile, so trade would be your best bet.where do I find some
Gentlemancorpse
Cannabis Chaotician
?
(RKS x Dirty Hippy) --> [ RKS x (Afgooey x Blockhead) ]
It's been unavailable from Bodhi for awhile, so trade would be your best bet.
Much appreciated Schwaggy... any chance there's any of your HS x Skunky Brewster cross still available? Or will be available again? I found out pretty quickly it won't be an easy one to find... I've found one other cross with it (HS x Ultraviolet OG) that I'm gonna dig through, but that's about it so far
Why is the F2 generation “wilder” than F1 or F3???
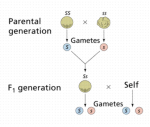
Many times, growers will say, “I love digging through F2s” or “I’ll try to find a male from the F2.” The realization that there is something peculiar about F2 seeds may seem puzzling. It rubs against the assumption that as a line is worked through subsequent generations, there should be a stabilizing force that funnels phenotypic variance with each new generation. Yet, we can see a marked difference in expressions during the F2 vs. F1 or F3. This concept was realized by Gregor Mendel during his experiments with peas.
While studying inheritance, Mendel bred one pea that was true-breeding for smooth peas (SS) with a wrinkled pea (ss) and observed that all of the resulting progeny in the F1 generation were smooth peas.
This is not shocking to us now that we understand the mechanics of inheritance. The dominant “smooth” trait ensures that all of the F1 phenotypes are smooth peas (Ss). It is worth noting that while all of our F1 peas have a smooth phenotype, their genotype now includes the recessive wrinkled allele (s).
When Mendel selected 2 peas from the F1 generation for breeding, the F2 generation produced 25% wrinkled peas.
Let’s take a look at a similar example using cannabis. Assume a breeder was working on plant height. This breeder wanted tall plants so they used their true-breeding for tall height Haze (SS) with a new short Afghan Skunk (ss) that had another trait of interest.
To the breeder’s satisfaction, the F1 generation produces all tall offspring (Ss). Our breeder would like to further refine the line to shore up this particular trait along with a few new traits acquired and selects two plants from the F1 to create F2.
Now, the breeder is surprised to see 25% of the F2 plants sporting a short height (ss). While the F1 phenos were very uniform, the F2 generation seems to be throwing a curveball. Our breeder culls the 25% shorties and selects from the remaining tall plants for the F3 generation.
The F3 offspring now assume the uniform tall height seen from the F1. The added benefit to the F3 generation is that we now have 50% true-breeding tall plants (SS). This would mean that if the breeder selects 2 (SS) plants for F4, the resulting genotypes would all be true-breeding for tall height.
This example is a good illustration of the difference in goals between growers-for-smoke(GFS) and breeders. Chuckers and GFS will be most interested in phenohunting populations with no explicit interest in the genotype. A breeder is a genohunter, testing and observing populations to create desirable genetic arrangements.
How traits from Grandparent plants can reemerge in the F2
The F2 generation can see the reappearance of traits from grandparent plants that were considered “gone”. To illustrate how this can happen, let’s see what our hypothetical breeder is up to now.
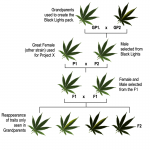
Schwaggy Z has a great female plant that he would like to use for a project (called Project X). He buys a pack of Black Lights (Black Domina x NL#1) to find a suitable male for his project. Schwaggy has never seen the individual Black Domina or NL#1 that went into this cross, so he’s just hoping to find a nice indica male.
After popping the entire pack, he observes all of the plants are green with no variegation. He selects a male plant from this parent stock (P) and mates him to his great female plant that is also green with no variegation. All of the F1 plants are green without variegation and our breeder is feeling pretty good about results so far. Schwaggy continues with selection from the F1 and breeds to the F2.
To his surprise, the F2 offspring now have green leaves with and without variegation as well as black leaves with and without variegation. Schwaggy is at a loss to explain where these traits came from and worries about some kind of pollen contamination. How can this be?
The breeder of the Black Lights used a Black Domina with black leaves that have variegation and a Northern Lights #1 with green leaves and no variegation. We’ll use:
G – dominant Green leaf
g – recessive black leaf
N – dominant NO variegation
n – recessive variegation
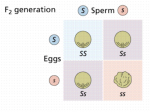
The Black Domina’s genotype is double recessive for both traits (ggnn) while the NL#1 is (GGNN). Let’s see how these grandparents breed to make the seeds Schwaggy popped to find a male.
Now we can see that the black variegation was “washed out” in the creation of the pack of seeds Schwaggy bought to find a male for Project X. Since he never saw the grandparents and only saw all green/no variegation plants, he never suspected the black/variegation traits were lurking.
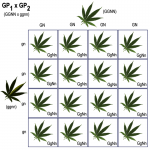
Schwaggy selects a male from this population (the pack of Black Lights) with the genotype (GgNn) to mate with his green/no variegated female (GGNN) to create F1 offspring.
All of the F1 offspring are green with no variegation, but the genotypes are now more diverse than the parent generation. Schwaggy selects a (GgNn) female and (GgNn) male to create F2 seeds.
To his shock, the F2 plants now produce:
~55% green leaves without variegation
~20% green variegated leaves
~20% black leaves without variegation
~5% black variegated leaves
The F2 generation saw the reappearance of a trait that was only seen from a grandparent plant and never manifested through the P or F1 generations.
With the aid of Punnett Squares, we are able to make sense of the F2 mystery.
It hasn't been released as a menu offering (still in testing).any chance there's any of your HS x Skunky Brewster cross still available?
This is really cool, and an awesome way to present it. I think the part about peas helped lure in my mushy brains... Thanks.Why is the F2 generation “wilder” than F1 or F3???
Many times, growers will say, “I love digging through F2s” or “I’ll try to find a male from the F2.” The realization that there is something peculiar about F2 seeds may seem puzzling. It rubs against the assumption that as a line is worked through subsequent generations, there should be a stabilizing force that funnels phenotypic variance with each new generation. Yet, we can see a marked difference in expressions during the F2 vs. F1 or F3. This concept was realized by Gregor Mendel during his experiments with peas.
While studying inheritance, Mendel bred one pea that was true-breeding for smooth peas (SS) with a wrinkled pea (ss) and observed that all of the resulting progeny in the F1 generation were smooth peas.
This is not shocking to us now that we understand the mechanics of inheritance. The dominant “smooth” trait ensures that all of the F1 phenotypes are smooth peas (Ss). It is worth noting that while all of our F1 peas have a smooth phenotype, their genotype now includes the recessive wrinkled allele (s).
When Mendel selected 2 peas from the F1 generation for breeding, the F2 generation produced 25% wrinkled peas.
Let’s take a look at a similar example using cannabis. Assume a breeder was working on plant height. This breeder wanted tall plants so they used their true-breeding for tall height Haze (SS) with a new short Afghan Skunk (ss) that had another trait of interest.
To the breeder’s satisfaction, the F1 generation produces all tall offspring (Ss). Our breeder would like to further refine the line to shore up this particular trait along with a few new traits acquired and selects two plants from the F1 to create F2.
Now, the breeder is surprised to see 25% of the F2 plants sporting a short height (ss). While the F1 phenos were very uniform, the F2 generation seems to be throwing a curveball. Our breeder culls the 25% shorties and selects from the remaining tall plants for the F3 generation.
The F3 offspring now assume the uniform tall height seen from the F1. The added benefit to the F3 generation is that we now have 50% true-breeding tall plants (SS). This would mean that if the breeder selects 2 (SS) plants for F4, the resulting genotypes would all be true-breeding for tall height.
This example is a good illustration of the difference in goals between growers-for-smoke(GFS) and breeders. Chuckers and GFS will be most interested in phenohunting populations with no explicit interest in the genotype. A breeder is a genohunter, testing and observing populations to create desirable genetic arrangements.
How traits from Grandparent plants can reemerge in the F2
The F2 generation can see the reappearance of traits from grandparent plants that were considered “gone”. To illustrate how this can happen, let’s see what our hypothetical breeder is up to now.
Schwaggy Z has a great female plant that he would like to use for a project (called Project X). He buys a pack of Black Lights (Black Domina x NL#1) to find a suitable male for his project. Schwaggy has never seen the individual Black Domina or NL#1 that went into this cross, so he’s just hoping to find a nice indica male.
After popping the entire pack, he observes all of the plants are green with no variegation. He selects a male plant from this parent stock (P) and mates him to his great female plant that is also green with no variegation. All of the F1 plants are green without variegation and our breeder is feeling pretty good about results so far. Schwaggy continues with selection from the F1 and breeds to the F2.
To his surprise, the F2 offspring now have green leaves with and without variegation as well as black leaves with and without variegation. Schwaggy is at a loss to explain where these traits came from and worries about some kind of pollen contamination. How can this be?
The breeder of the Black Lights used a Black Domina with black leaves that have variegation and a Northern Lights #1 with green leaves and no variegation. We’ll use:
G – dominant Green leaf
g – recessive black leaf
N – dominant NO variegation
n – recessive variegation
The Black Domina’s genotype is double recessive for both traits (ggnn) while the NL#1 is (GGNN). Let’s see how these grandparents breed to make the seeds Schwaggy popped to find a male.
Now we can see that the black variegation was “washed out” in the creation of the pack of seeds Schwaggy bought to find a male for Project X. Since he never saw the grandparents and only saw all green/no variegation plants, he never suspected the black/variegation traits were lurking.
Schwaggy selects a male from this population (the pack of Black Lights) with the genotype (GgNn) to mate with his green/no variegated female (GGNN) to create F1 offspring.
All of the F1 offspring are green with no variegation, but the genotypes are now more diverse than the parent generation. Schwaggy selects a (GgNn) female and (GgNn) male to create F2 seeds.
To his shock, the F2 plants now produce:
~55% green leaves without variegation
~20% green variegated leaves
~20% black leaves without variegation
~5% black variegated leaves
The F2 generation saw the reappearance of a trait that was only seen from a grandparent plant and never manifested through the P or F1 generations.
With the aid of Punnett Squares, we are able to make sense of the F2 mystery.
MacGydro
Gum Wrapper Grows
Of the Chem cuts, it sounds most like the SKVA. The lemons comment isn't something I get from her nose, but everything else you described sounds similar to her. Some speculate that OG Kush is a SKVA S1, so if that holds, there could be some lemon in there you might be able to tease out or you may have had an S1.
Thanks so much for taking the time! Yeah, it was definitely lemons first, then straight gasoline. Figured you'd be a good person to ask, since you have the different cuts for comparison. I hear a lot of descriptions of "gas" from people lately, but wonder if it's actually a gasoline smell, or they're just saying "fire" in another way.
Anyway, I've got a Skunky VA x Skunky D inside, at about 7 1/2 weeks so far, and a petite, hungry little Skunky VA outside, looking about 4 or 5 weeks along so far. I haven't sniffed the one inside lately, but definitely get skunk notes from the VA outside so far. I'm looking forward to trying both either way, and even put a male VA/D outside to play with the VA, and a few other gals.
Thanks for all the info on the dissolved oxygen! Waaay over my head figuring out specific numbers, like I kinda figured, so I'll just hope the levels are sufficient, and be happy I don't lose anything to root rot lol
Gentlemancorpse
Cannabis Chaotician
It hasn't been released as a menu offering (still in testing).
Cool, I'll be keeping an eye out for it then... hope your testing goes well haha
How do I as recreational chucker avoid bottlenecking myself? Can this be done without infusing new genetics?
I have made some f1s and am debating a path forward if any. I am not necessarily on a targeted breeding project other than to reduce the risk of bottlenecking myself, essentially selectively chucking to the point of loosing some important allele that is unknown to me because I based of phenotypic expression (please forgive if that is not the correct terminolgy). What if the trait I key in on is actually a genetic flaw?
This leads me to think the best option to capture the whole of a f1 chuck is to run a 32 plant open pollination run. Ideally it would split 50/50 male/female but that probably wont happen then if I start pulling females and popping more beans searching for males am I really diversifying the gene pool any? I would be selecting out the population even if not intentional?
Many individual questions but hopefully the broader answer can entertain a wider audience?
I can hardly hold the anticipation.
I have made some f1s and am debating a path forward if any. I am not necessarily on a targeted breeding project other than to reduce the risk of bottlenecking myself, essentially selectively chucking to the point of loosing some important allele that is unknown to me because I based of phenotypic expression (please forgive if that is not the correct terminolgy). What if the trait I key in on is actually a genetic flaw?
This leads me to think the best option to capture the whole of a f1 chuck is to run a 32 plant open pollination run. Ideally it would split 50/50 male/female but that probably wont happen then if I start pulling females and popping more beans searching for males am I really diversifying the gene pool any? I would be selecting out the population even if not intentional?
Many individual questions but hopefully the broader answer can entertain a wider audience?
I can hardly hold the anticipation.

MacGydro
Gum Wrapper Grows
Many individual questions but hopefully the broader answer can entertain a wider audience?
I can hardly hold the anticipation.
Hey, if it helps at all, I can hardly contain my anticipation either, but I'll certainly help you hunt through any F2s or subsequent seeds you don't feel like lol
Good luck buddy!!
The term bottlenecking refers to contraction in genetic diversity of a population due to limited population. This can be a great thing in the case of a targeted breeding project as your goal is to create a uniform strain of plants that exhibit little genetic variation in higher generations (either filial F3+, selfed S2+). In this context, bottlenecking is providing the stabilizing force to the intended line.How do I as recreational chucker avoid bottlenecking myself? Can this be done without infusing new genetics?
If you were more interested in preservation of specific lines, then bottlenecking can be seen as something to avoid. You’d want to maintain the genetic diversity within a strain’s larger gene pool encompassing as many phenotypic expressions as the genotype possibilities allow.
Depending on what you aim to achieve, bottlenecking can be friend or foe. If your worry is more general, in the sense that over time you want to leave options open in a line you continually select to take to the next filial generation, then try to include a wider pool of selections when making your next seeds. Full open pollination will be the most effective process to achieve this. The pressure that is exerting the genetic contraction is from smaller mating populations, so widening your criteria for selections can help keep genetic diversity intact. Instead of choosing a single male, you can choose the best representatives of each main pheno you observe, mix their pollen and pollinate corresponding female exemplars of these phenos. In this way, even with just 2 males (one leaning from either parent) you’ll ensure some level of genetic diversity moving forward.
If you’re working with something like an F10+ and you feel all phenos are hopelessly uniform, you may consider help from an outside line. If your worry is that this infusion of new genetics will crash the genetic party too hard, then I’d suggest a backcrossing to either BX2 or BX3. Then, selecting a male from the BX2-3 that you can then introduce to your original line. To just take a totally different male from an outside line and use it would indeed flood your original work with too much new influence.
What backcrossing will do, is for every BX generation, you will halve the influence of the new genetics. The first outcross will give you 50%original strain/50%new strain. The first backcrossing will then give you 75%original/25%new. The second backcross gives you 88%original/12%new, and the third BX (cube) gives you 94%original/6%new. Notice that the contribution of the new strain is halved at every new backcrossed generation. Depending on how much influence you’d like to introduce to your original strain that has become bottlenecked, you can backcross until you are comfortable that the new infusion will add genetic diversity in a way that does not compete with your original strain.
You can further ensure this limited infusion is consonant with your original line by choosing a related line for the outcrossing to begin the BX process. For example, if your original line is Northern Lights, then you can select an Afghan male from another line to use in your initial outcross. Now, whenever you finish your backcrossing to the targeted NL, you’ll be able to select a male from the BX to pollinate your original strain’s females to introduce a small infusion of related genetics in such a way as to open up genetic diversity without destroying the qualities that drew you into the original strain.
I tried to mitigate the loss of such “hidden” recessive alleles when doing the HAOG BX. The way I tried to accomplish this is through what I called “polyandric backcrossing”. It is essentially doing 3 simultaneous backcrossings using a different male for each and tracking the resulting females to ensure that the traits that encompass the HAOG phenotype wasn’t lost due to loss of a recessive allele in the selection process. If a particular male seemed to produce females lacking some quintessential HAOG trait, then I knew a recessive trait was being smothered and discontinued that specific BX line. I’ll post the full write up of that process soon.I have made some f1s and am debating a path forward if any. I am not necessarily on a targeted breeding project other than to reduce the risk of bottlenecking myself, essentially selectively chucking to the point of loosing some important allele that is unknown to me because I based of phenotypic expression (please forgive if that is not the correct terminolgy). What if the trait I key in on is actually a genetic flaw?
Genetic flaws are usually double recessive traits. A trait usually becomes considered a flaw if either its expression hinders the plants normal growth processes or if the trait expresses rarely such that it is assumed to be some aberration to the “normal” expressions of a strain. I would treat the “flaw” as a double recessive and breed/select accordingly until observations suggests otherwise.
If your fear is that you’ll inadvertently lose something through selection, then open pollination of as many males/females is the most you can do if limited to only using the original line. I would conceptualize the open pollinations as keeping the gene pool as diverse as the original starting material allows, as you are limited to within the confines of whatever that line’s starting gene diversity afforded.This leads me to think the best option to capture the whole of a f1 chuck is to run a 32 plant open pollination run. Ideally it would split 50/50 male/female but that probably wont happen then if I start pulling females and popping more beans searching for males am I really diversifying the gene pool any? I would be selecting out the population even if not intentional?
Awesome! Thanks for giving Schwaggy Seeds a shot. There's info about them here:Schwaggy SeedsI can hardly hold the anticipation.
HOGBACKMAGIC14
In Bloom
just got my order the other day i am going to grow these outside next year unless i have time this winter
GLG came through again i got my schwaggy gear nice looking seeds and a awsome beer coozy
thanks GLG and Schwaggy P keep up the good work and always enjoy your posts brother
GLG came through again i got my schwaggy gear nice looking seeds and a awsome beer coozy
thanks GLG and Schwaggy P keep up the good work and always enjoy your posts brother

One of the main reasons I asked the questions is I have 2 polyembryotic females. I dropped 9 beans from another chuckers BX and came up with 11 plants. I also had a twin with the f1 generation (less than 20 se seeds).
I feel like I have been handed an opportunity for a little experiment, unfortunately (I prefer regs) they are both female, I have never attempted reversing a plant. I feel like this added layer of copmplexity could skew the result.
The twins are not from the same seed, the weaker one of each did not make it.
From what I understand there are 2 process and 5 genetic markers (a brief review of data on the phenomenon in citrus. Characterization of genes associated with polyembryony and in vitro somatic embryogenesis in Citrus and Phenomenon of polyembryony. Genetic heterogeneity of seeds both abstracts quickly passed over my head)
Before the beans were popped I was planning a polyandric filial generation using the limited pool, in total 5 male 5 female, 9 from this seed pop and 1 from a previous run. This lead to the bottlenecking question as I am not sure if I am hunting a flaw or a feature.
Two of the clones are barely rooted and I am still going to have to take cuts of them (after clearing out a garden issue I am having) so it will be a minute before I attempt any of this.
WWSD? Should I attempt the reversal or hunt for male and female twins from my f1s?
I feel like I have been handed an opportunity for a little experiment, unfortunately (I prefer regs) they are both female, I have never attempted reversing a plant. I feel like this added layer of copmplexity could skew the result.
The twins are not from the same seed, the weaker one of each did not make it.
From what I understand there are 2 process and 5 genetic markers (a brief review of data on the phenomenon in citrus. Characterization of genes associated with polyembryony and in vitro somatic embryogenesis in Citrus and Phenomenon of polyembryony. Genetic heterogeneity of seeds both abstracts quickly passed over my head)
Before the beans were popped I was planning a polyandric filial generation using the limited pool, in total 5 male 5 female, 9 from this seed pop and 1 from a previous run. This lead to the bottlenecking question as I am not sure if I am hunting a flaw or a feature.
Two of the clones are barely rooted and I am still going to have to take cuts of them (after clearing out a garden issue I am having) so it will be a minute before I attempt any of this.
WWSD? Should I attempt the reversal or hunt for male and female twins from my f1s?
Got a question for ya. Take a cut of wedding cake. Same cut shared a thousand times but the results are much different based on how/where its grown. Even using same lights/nutes/media in four grows turns into four different looks, g/w, lab test scores. Hows this explain that? Mendel says it shouldt be the case. Are dom traits not important for cannabis?
Frimpong
🔥Freak Genetics🔥
There are more variables than we can even comprehend I bet . JmhoGot a question for ya. Take a cut of wedding cake. Same cut shared a thousand times but the results are much different based on how/where its grown. Even using same lights/nutes/media in four grows turns into four different looks, g/w, lab test scores. Hows this explain that? Mendel says it shouldt be the case. Are dom traits not important for cannabis?
stanknugzz77
CHOOSE YOUR TITLE
I know that your question is directed towards @Schwaggy P, but my understanding: Genotype is the only constant with cuttings (clones). Environment plays a huge role in phenotypic expressions. My guess would be that unless someone is growing in a laboratory setting, they are failing to recreate the exact environment down to a T. Temperature, humidity, nutrient availability and uptake etc all make up the cuts environment, thus leading to different phenotypic expressions. Again, this is just my understanding of the science behind it. I am certain that @Schwaggy P will have a better grasp on it. Positive vibes...Got a question for ya. Take a cut of wedding cake. Same cut shared a thousand times but the results are much different based on how/where its grown. Even using same lights/nutes/media in four grows turns into four different looks, g/w, lab test scores. Hows this explain that? Mendel says it shouldt be the case. Are dom traits not important for cannabis?
~nugzz
Similar threads
- Replies
- 51
- Views
- 1K
- Replies
- 144
- Views
- 6K